A study in the journal water air and soil pollution by the university of hong kong reported that artificial acid rain with a ph of 3 5 could corrode.
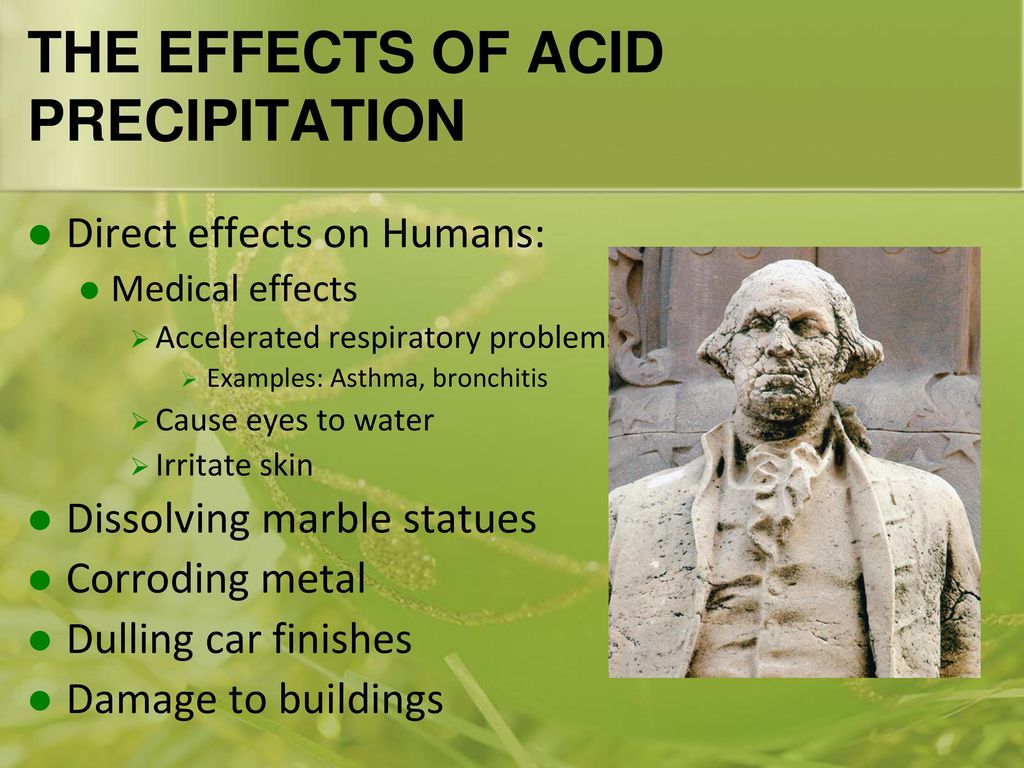
Why does acid rain dissolve statues made of marble.
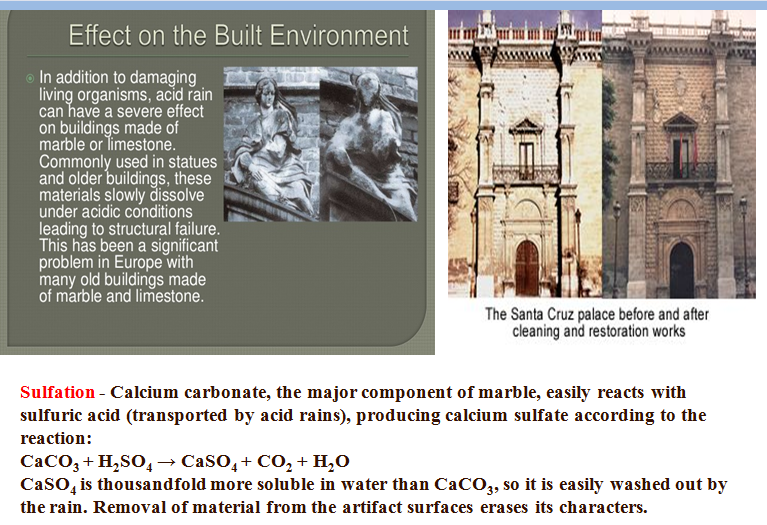
Many monuments are made from limestone marble and bronze materials that can be altered or slowly dissolved by acid precipitation.
The reaction between caco and h so acid is caco s h so aq caso aq co g h o l caso is slightly soluble in water.
In exposed areas of buildings and statues we see roughened surfaces removal of material and loss of carved details.
When sulfurous sulfuric and nitric acids in polluted air react with the calcite in marble and limestone the calcite dissolves.
Stone surface material may be lost all over or only in spots that are more reactive.
The marble has caco as the major component.
Slowly is the key word of course.
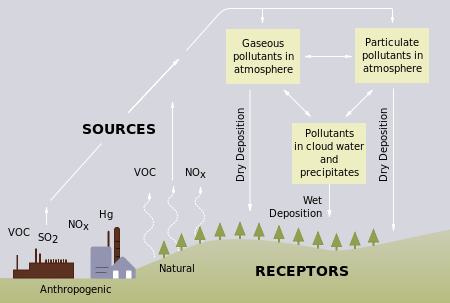
Acid precipitation affects stone primarily in two ways.
How does acid precipitation affect marble and limestone buildings.
In exposed areas of buildings and statues we see roughened.
Vulnerable metals include bronze copper nickel zinc and certain types of steel.
The acids in acid rains can react with caco by producing soluble salts.
That s why acid rain dissolves statues made of marble.
No one expects the washington monument to melt into a toothpick but acid rain damage may slowly add up for our beloved icons.